Cleaning/Sanitization Verification with ATP in Orlando, FL.
ATP Cleaning Verification and Monitoring Systems offer rapid evaluation of biological contamination on surfaces. Primarily, ATP Cleaning Verification and Monitoring Systems are used to test surfaces after cleaning/sanitization/sanitation efforts are completed to gauge the effectiveness of the performed remedial work.
ATP Cleaning Verification and Monitoring Systems are used in a variety of industries including:
Healthcare
- Hospitals
- Doctor’s offices
- Mobile testing/diagnostics vehicles
- Ambulances
Education
- Universities
- K-12 schools
Assisted Care Facilities
- Childcare
- Adult/Senior daycare
- Nursing homes
Hospitality
- Hotels/motels
- Cruise ships
- Fitness centers
Food Service
- Restaurants
- Supermarkets
- Food/beverage processing
- Cannabis processing
Office Environments
- Call centers
- Break rooms
- Restrooms
- Elevators
Environmental Remediation
- Category-3 water
- Crime scene/trauma

Why is testing of surfaces important?
Infectious diseases are disorders caused by organisms. Infectious diseases are spread by direct contact, indirect contact, insect bites, and food contamination.
Fomite transmission refers to the transmission of infectious diseases by germs left on objects. A fomite is any inanimate object (such as door knobs, faucet handles, flooring, furniture, hand rails, tabletops, utensils, toys, etc.) that, when contaminated with or exposed to infectious agents (bacteria, viruses, fungi, and parasites), can transfer disease to a new host. When you touch the contaminated object, you can pick up the germs.
Testing surfaces for biological contamination can help identify contamination loads on high-touch surfaces. The identification of ATP on surfaces may also serve as a surrogate for microbial contamination that may not be easily tested for in the field.
What is ATP?
Adenosine Triphosphate (ATP) is the universal energy molecule found in all living things. Animals, plants, bacteria, and fungi (molds/mushrooms/yeasts) cells produce ATP in the mitochondria (powerhouse) of cells. ATP is used as a source of chemical energy for cells.
How is ATP used in ATP cleaning verification and monitoring systems?
ATP testing devices contain a natural enzyme found in fireflies. This enzyme produces a bioluminescence (light-producing) reaction when it contacts ATP. Using the bioluminescence emitted by the reaction, the luminometer measures ATP encountered and displays/saves the results numerically in the form of Relative Light Units (RTU).
Why is using an ATP cleaning verification and monitoring system a good metric for evaluation of biological contamination?
“While many methods exist for evaluating cleanliness, ATP bioluminescence is the only method that combines quantitative data collection with scientific measurement and still delivers speedy results.”1
Speed– Real-time analysis yields actionable information in seconds.
Linearity– There is a direct, proportional, straight-line relationship between RLU and ATP.
Repeatability– There are dependable results with a low coefficient of variation (CV).
Sensitivity– The is an extremely low Limit of Detection (LoD) allowing for identification of low levels of ATP above background noise.
Accuracy– Based on a comparative study of commercial ATP Hygiene Monitoring Systems2, the ATP system used by FSG Inspections is the closest to 100%, thus the least variable and most accurate in the study.
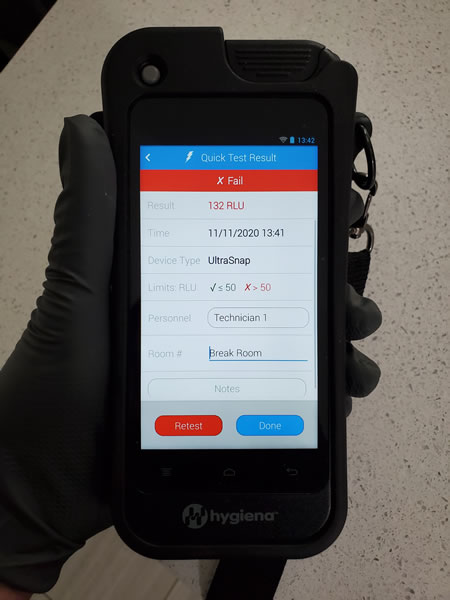
What equipment does equipment does FSG Inspections use?
FSG Inspections uses the EnSURE™ Touch advanced cleaning verification and monitoring system by Hygiena. Hygiena is the global industry leader in ATP cleaning verification and monitoring systems. The EnSURE™ Touch is Hygiena’s next-generation, state-of-the-art ATP system that collects, analyzes, and reports ATP and site data.
In order to have robust quality controls, FSG Inspections uses the Hygiena CalCheck LED Calibration Verification system in addition to the EnSURE™ Touch in-built startup calibration check for secondary on-site positive and negative calibration checks.
Samples are collected by UltraSnapTM and SuperSnapTM surface samplers based on sensitivity requirements of the Client.
